Trigonelline Reverses Lung Scarring in Rats.
This study evaluates trigonelline, a natural compound, to remedy lung scarring in rats. It was tested alone or combined with Pirfenidone (Pirf), a clinically approved, but not well-tolerated drug to treat lung damage.
“Following the COVID-19 outbreak and the limitation of the current therapies for PF, the burden of pulmonary fibrotic disease is expected to be considerable.”
Key Points:
The inclusion of TRG demonstrated marked improvements in induced lung damage models.
- Reversal of BLM-induced lung damage.
- Reduction in harmful collagen deposits and inflammatory cells.
- Improvement in cellular aging, recycling, and programmed cell death.
- Increased animal survivability.
“The current study verifies TRG’s prophylactic and antifibrotic effects against BLM-induced PF via targeting multiple signaling. TRG and Pirf combination may be a promising approach to synergize Pirf antifibrotic effect.”
Trigonelline Studied in vivo.
Ninety-five adult male Sprague–Dawley rats were chosen for this study and randomized into 7 groups. Bleomycin (BLM) was used to induce scarring of the lung tissue; TRG or Pirf treatment was administered orally.
- Sham/Control (n = 15): saline
- TRG (n = 15): TRG (50 mg/kg/day) 3 days before saline for 28 days
- BLM (n = 20): BLM (5 mg/kg in saline)
- BLM + TRG prophylactic-(P) (n = 15): TRG 3 days before BLM dose + TRG for 28 days
- BLM + Pirf therapeutic-(T) (n = 15): BLM + Pirf (100 mg/kg/day) from days 8 – 28
- BLM + TRG therapeutic-(T) (n = 15): BLM + TRG from days 8 – 28
- BLM + TRG/Pirf therapeutic-(T) (n = 15): BLM + TRG + Pirf) from days 8 – 28
“Lung function parameters in patients with PF, such as forced vital capacity and total lung capacity were significantly lower in men relative to women.”
Trigonelline Improves Lung Tissue and Reverses Damage.
TRG treated rats showed improved lung cell health compared to the BLM group in tissue samples. The data showed:
- Reduced cellular damage.
- Partially restored lung cells.
- TRG administered chronically, performed slightly better than therapeutic TRG at PF. Overall results are similar to clinically approved Pirf.
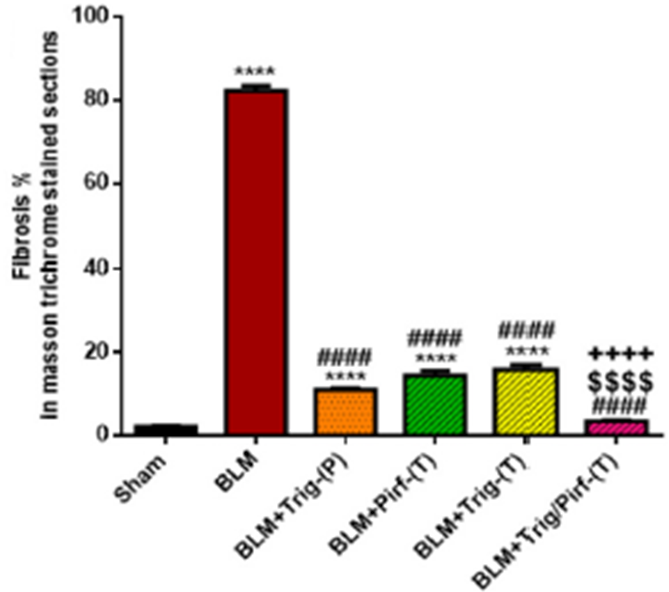
This figure shows the percentage of scarring following PF induction and treatment. Co-administration of TRG/Pirf (red striped), showed the largest decrease in damage. Therapeutically administered TRG (yellow) and Pirf (green) yield similar results while chronically dosed TRG (orange) showed slight improvement in comparison.
“TRG either as a prophylactic or therapeutic supplement induced a reduction in lung index, total protein as well as lung injury biomarkers similar to Pirf revealing a significant cytoprotective effect of TRG on lung cells.”
Trigonelline Reduces Inflammatory Cell Counts.
Researchers found that total immune cell counts were significantly elevated in BLM-induced rats.
- As a preventative treatment, TRG significantly reduced the number of inflammatory cells compared to the BLM group.
- Pirf +TRG significantly enhanced this reduction compared to either treatment alone, bringing counts back to normal levels.
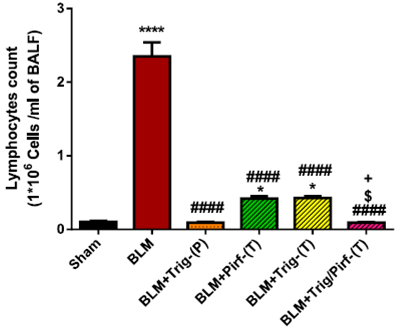
Chronically dosed TRG (orange) and Pirf/TRG (pink) combination therapy decreased the total immune cell counts to normal levels (black).
“Overall lung inflammation scores were significantly decreased in BLM + TRG/Pirf-(T) group when compared to Pirf, and therapeutic TRG groups.”
Trigonelline Reduces Cellular Aging.
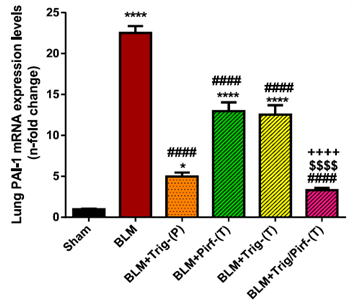
- BLM increased the expression of genes promoting scarring. TRG/Pirf most effectively suppressed these genes and showed significant increase in cellular recycling.
- TRG reduced markers of cellular death and aging.
This figure illustrates the increase of gene PAI-1 in BLM rats (red) which is associated with tissue scarring. This is effectively decreased by chronically administered TRG (orange) and combination therapy (pink).
“Several significantly upregulated genes in BLM rats were markedly reduced in TRG-treated rats.”
Trigonelline Increases Survivability.

This figure depicts a mortality rate of 30% in BLM-induced rats (red) and % in rats treated with Pirf (blue). No mortality was observed in the remaining groups.
“Interestingly, TRG and Pirf combination therapy potentiated the antifibrotic activity for pF treatment to a greater extent that either treatment alone.; combination therapy may be a promising avenue to enhance the antifibrotic effect of Pirf”
Conclusions
“We can conclude that the antifibrotic effect of TRG in mitigating PF is mainly attributed to its immunomodulating, and anti-inflammatory effects.”

